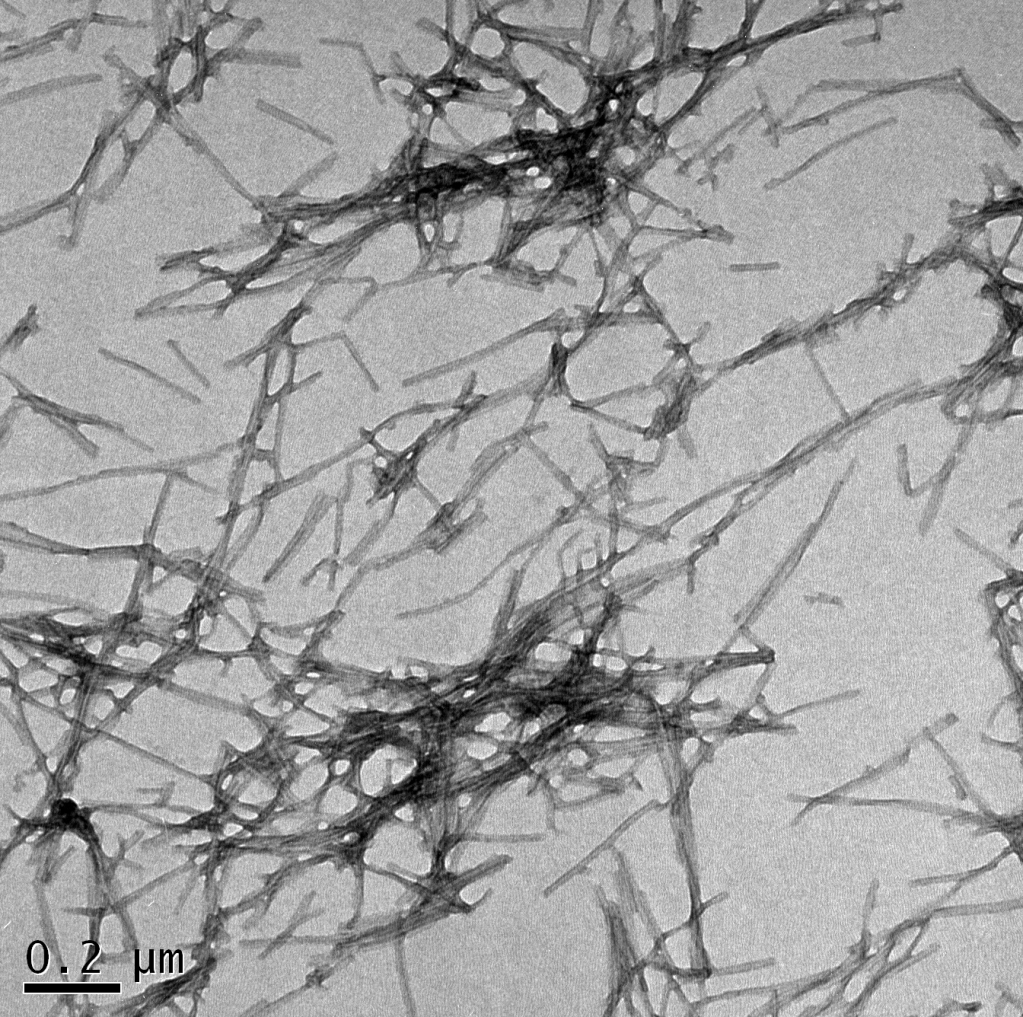
Mechanism of α-synuclein amyloid fibril formation
Amyloid fibrils of the intrinsically disordered protein α-synuclein are the main component of Lewy bodies, intra-neuronal inclusions that characterize the pathology of Parkinson’s disease. We aim to understand the mechanism of transformation of α-synuclein from its disordered monomeric state in solution into the highly ordered, β-sheet rich amyloid state. In collaboration with the company Lundbeck, and funded by the Michael J Fox Foundation, we are also aiming to exploit our mechanistic understanding for the development of novel therapeutic methods for Parkinson’s disease and multiple system atrophy.
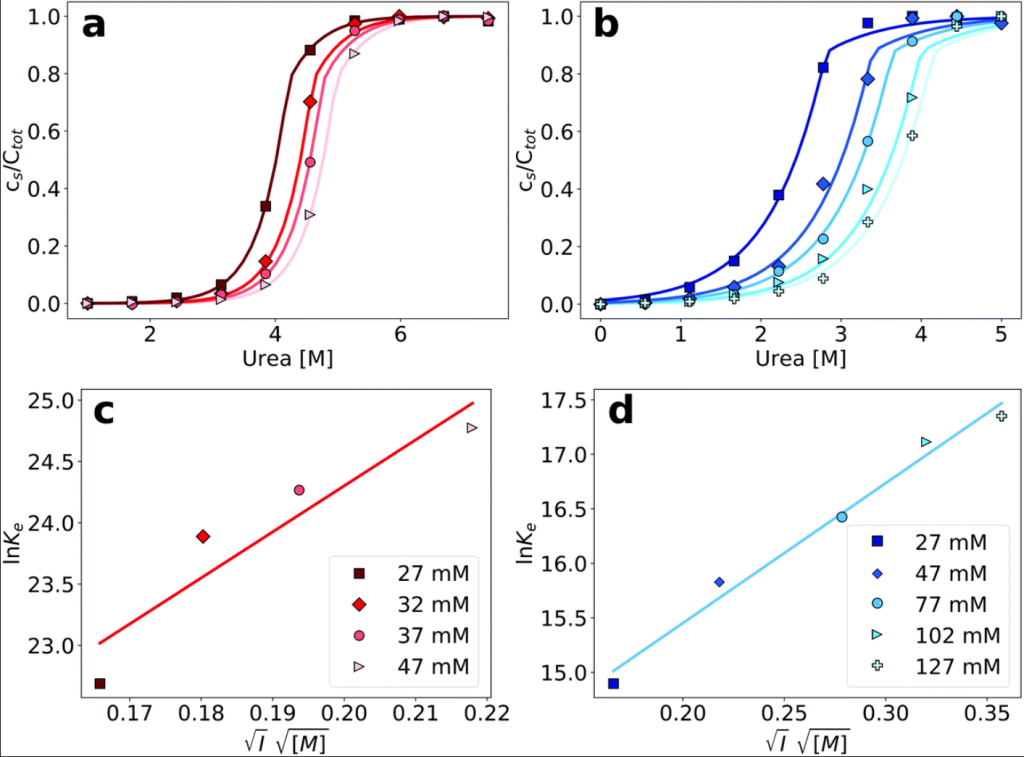
The thermodynamic stability of amyloid fibrils
Amyloid fibrils are generally thought to be thermodynamically quite stable, but exactly how stable they are, relative to the soluble state, has been surprisingly difficult to quantify in the past. We are developing biophysical methods to quantify the thermodynamic stability of amyloid fibrils and how it is affected by solution conditions and changes in amino acid sequence. In the context of a project funded by the Lundbeck Foundation, we investigate the link between thermodynamic stability of fibrils and their susceptibility towards biological clearance mechanisms.
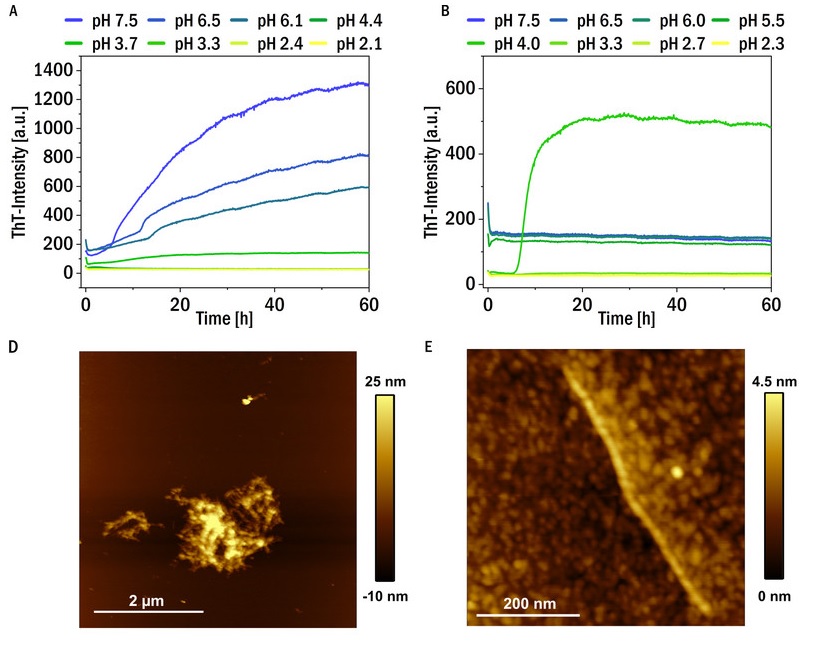
Mechanism of pathological aggregation of immunoglobulin light chain
Protein aggregation can not only cause neurodegenerative diseases, but can also damage organs other than the brain. In certain types of cancers of immune cells, such as multiple myeloma, large concentrations of antibody fragments (e.g. immunoglobulin light chains) can be produced and released into the blood. This can in some cases lead to the aggregation and deposition of these proteins in the form of either amorphous aggregates or amyloid fibrils. This deposition, in turn, can lead to severe organ damage, for example of the heart or kidney. We are trying to understand what differentiates the immunoglobulin light chains that are aggregating and causing damage from those that are highly soluble and simply get excreted with the urine.
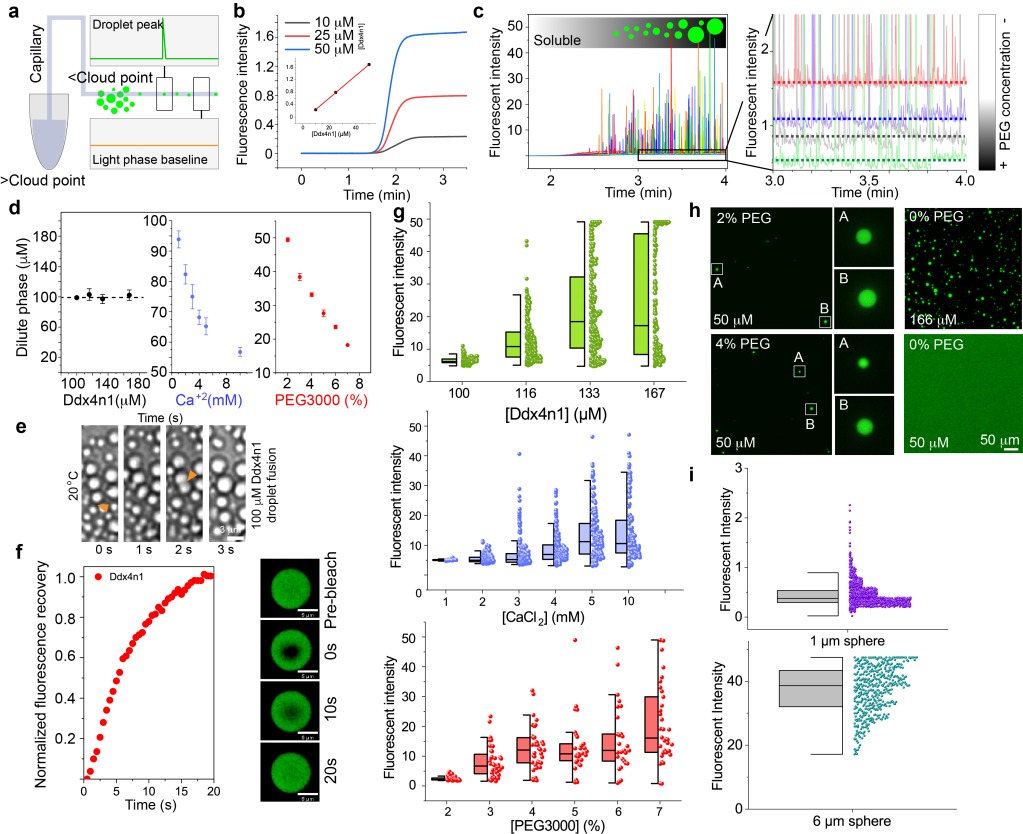
Liquid-liquid phase separation of proteins
Liquid-liquid phase separation (LLPS) of proteins corresponds to the spontaneous separation upon external stimulus, of a protein solution into protein-rich droplets within a dilute background. Protein LLPS is thought to play a role in a wide range of relevant processes, ranging from the formation of membrane-less organelles in living cells to protein crystallization and the formulation of biologics in the pharmaceutical industry. Furthermore, many proteins that form amyloid fibrils in the context of neurodegenerative diseases can also undergo LLPS, which could be a pre-cursor in some cases to amyloid fibril formation. We are interested in the factors that drive LLPS and that lead to the conversion of reversible droplets into irreversible amyloid fibrils.
Functional self assembly of proteins and peptides into useful materials
Peptide and protein aggregates are not only associated with disease, they also have remarkable mechanical, structural and functional properties. Nature exploits these properties in the case of functional amyloid fibrils, for example for bacterial or fungal adhesion and melanin biosynthesis. We are aiming to understand and control the self-assembly of peptides and proteins, which are not linked to disease, into interesting and useful materials. Our approach is hereby systematic: we want to understand the assembly mechanisms in order to be able to rationally choose the conditions for the assembly of the materials. The aim of our targeted approaches to protein based materials is to render them ever more sustainable and minimize their carbon footprint.

Biophysical studies of plant seed storage proteins for food applications
In collaboration with researchers at the University of Copenhagen and at Le Mans University, we are working on a large project, SEEDFOOD, in which we aim to isolate, purify and study the main seed storage proteins from rapeseed. SEEDFOOD, which is funded by the Novo Nordisk Foundation, aims to convert the rapeseed storage proteins, which are currently used mostly for animal feed, into high quality protein fit for human consumption. In order to achieve that goal, a lot of basic protein biophysics will need to be done on these understudied proteins, and that is going to be our group’s contribution.

High throughput biophysics
We are also interested to push up the throughput and push down the sample consumption of biophysical experiments of biomolecular interactions. We develop methods in this area that are either based on commercially available experimental platforms, or else on our home-built microfluidics chips.
